Stephen L. Bearne
Professor

Email: stephen.bearne@dal.ca
Phone: 902-494-1974
Mailing Address:
Sir Charles Tupper Medical Building
PO Box 15000
Halifax, Nova Scotia, Canada B3H 4R2
Education
- PhD, University of Toronto
- MDCM, McGill University
Academics Positions
- Department member since 1996
- Department Head (2012 - 2022)
- Member, Protein Assembly Research Team
- Member, BioActives CREATE Training Program
Research Topics:
Enzyme catalysis and protein engineering: transition state analogues; enzyme inhibition; bio-organic reaction mechanisms; organic synthesis; biochemical recognition; protein modifications; active site architecture; enzyme evolution; proteomics
Research
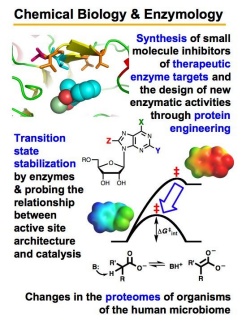
Our research program has three major goals: (1) to understand the nature of the protein-ligand interactions that contribute to stabilization of reactive intermediates and transition states, relative to the ground state, (2) using this information to engineer new catalytic activities and develop tools for identification of specific enzymes in complex proteomes, and (3) to understand the chemical mechanism of enzymes that are of therapeutic interest. This research is ideal for someone with a passion for biological chemistry, enzyme mechanisms, and enzyme catalysis.
FUNDAMENTAL ENZYMOLOGY: Mechanism, Structure, Function, & Evolution
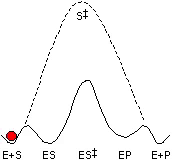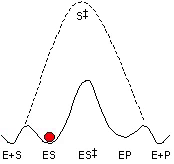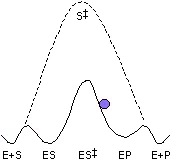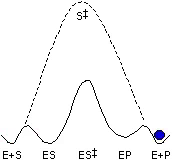
Mandelate racemase (MR) is a member of the enolase superfamily of enzymes and catalyzes the rapid carbon-hydrogen bond cleavage of a carbon acid substrate with a relatively high pKa value. We are studying MR as a paradigm for understanding how enzymes overcome the problem of carbon acidity, a common feature of many biological reactions. Using new transition state analogue inhibitors that we have developed, in combination with site-directed mutagenesis and biophysical techniques, we are studying the nature of the protein-ligand interactions that stabilize the altered substrate in the transition state. We are also conducting protein engineering and directed evolution to alter the substrate specificity of MR, and we are conducting investigations to understand the evolution of complexity in enzyme structure.
ENZYME TARGETS IN DISEASE: Neglected Tropical Diseases, Cancer, & Antibiotics
The enzyme cytidine 5'-triphosphate synthase (CTPS) belongs to a family of enzymes known as the glutamine amidotransferases and catalyzes the conversion of UTP to CTP. Inhibition of CTPS activity could offer a means of ameliorating a variety of diseases, including cancer, viral infections, and protozoal infections. Our goals are to understand how the allosteric effector, GTP acts to promote glutamine hydrolysis, and to develop specific inhibitors of GTP-dependent activation of CTPS. We are also studying the enzymology of racemases with the goal of developing specific and potent inhibitors. Racemases under investigation include the prostate cancer biomarker alpha-methylacyl-CoA racemase, and those racemases involved in generating D-amino acids (antibacterial targets).
PROTEOMICS
Studies are focused on developing reagents for proteomic profiling of enzymatic activities.
DEVELOPING YOUR TOOL BOX
Graduate training in the Bearne Lab is designed to develop your critical and analytical thinking skills. Students have the opportunity to develop technical skills in a vast number of areas, including organic synthesis and protein chemistry, physical organic laboratory techniques, molecular modeling, enzyme kinetics, CD spectroscopy, microbiology, molecular biology, site-directed mutagenesis, isothermal titration calorimetry, macroion mobility spectrometry, NMR spectroscopy, chromatography (e.g.,HPLC & FPLC), and proteomics. This broad set of techniques is a great asset for students interested in exploring the exciting frontiers at the interface of modern biology and chemistry.
EDI Statement
The Bearne Enzymology Lab supports the principles of equity, diversity, and inclusiveness. Highly qualified trainees with an interest in enzymology and related areas of organic chemistry and biochemistry are welcome, regardless of ethnicity, gender identity, sexual orientation, religion, age, or physical (dis)ability. The Bearne Lab provides a collegial learning environment that fosters skill development and critical thinking, and recognizes the valuable contributions that can arise from a diverse set of trainees with a wide range of perspectives and unique ideas.
We thank the following agencies for past and present grants in aid of our research:
NSERC, CIHR, NSHRF, the Beatrice Hunter Cancer Research Institute, the Dalhousie Medical Research Foundation, and the Prostate Cancer Research Foundation
Keywords:
enzymology, enzyme kinetics and inhibition, chemical biology, organic synthesis, proteomics. Techniques: site-directed mutagenesis, protein & modification, HPLC, FPLC, CD, UV-vis, NMR, 2D gel electrophoresis, ITC, macroion mobility spectrometry
Lab Video
Current Lab Members
| Liam Bystrom | Grad Student (PhD, biochem) |
|---|---|
| Meghan Hamilton | Grad Student (PhD, biochem) |
| Katherine Kim | Co-op (chem) |
| Zulqi Memon | Honours Student (2025) |
| Julia Moncrief | Grad Student (MSc, chem) |
| Olivier Verville | Grad Student (MSc, biochem) |
Publications
- Fanaee, S., Zabihihesari, A., Ning, T., Austin, W., Wolverton, M., Leung, B.M., Bearne, S.L., Li, J., Filiaggi, M.J., Adibnia, V.* (2025) Advanced polyelectrolyte complexes for ultrafast hemostasis and blood superabsorption. Acta Biomater. 209, 202-305. [Article] [PubMed]
- Bearne, S.L.* (2025) A practical consideration for the substrate concentration when determining IC50values for enzyme inhibition, Biochem. Cell Biol. 103, 1-10. [Article] [PubMed]
- Ramesh, K., Bearne, S.L.* (2025) Convenient alternative synthesis of Malassezia-derived virulence factor malassezione and related compounds. Beilstein J. Org. Chem. 21, 1730-1736. [Article] [PubMed]
- Sorbara, N.T., Black, A.K.A., Bearne, S.L.* (2025) Bulky substrates of isoleucine 2-epimerase: α-neopentylglycine and NV-5138. Bioorg. Med. Chem. Lett. 122, 130160. [Article] [PubMed]
- Kumar, H., Kuehm, O.P., Aboushawareb, S.A.E., Rafiei, A., Easton, N.M., Bearne, S.L.* (2025) An active-site Brønsted acid-base catalyst destabilizes mandelate racemase and related subgroup enzymes: Implications for catalysis. Biochemistry 64, 666-677. [Article] [PubMed]
- Bearne, S.L. (2024) Biochemical communication between filament-forming enzymes: Potential regulatory roles of metabolites in enzyme co-assemblies with CTP synthase. Bioessays 46, e2400063. [Article] [PubMed]
- McGary, L.C., Fetter, C.M., Gu, M., Hamilton, M.C., Kumar, H., Kuehm, O.P., Douglas, C.D., Bearne, S.L.* (2024) Interrogating L-fuconate dehydratase with tartronate and 3-hydroxypyruvate reveals subtle differences within the mandelate racemase-subgroup of the enolase superfamily. Arch Biochem Biophys. 754, 109924. [Article] [PubMed]
- McGary, L.C., Regan, G.L., & Bearne, S.L.* (2023) Reactive architecture profiling with a methyl acyl phosphate electrophile. Biochim. Biophys. Acta Proteins Proteom. 1871, 140945 [Article] [PubMed]
- Bearne, S.L.* (2023) Design and evaluation of substrate-product analog inhibitors for racemases and epimerases utilizing a 1,1-proton transfer mechanism. Methods Enzymol. 690, 397-444. [Article] [PubMed]
- Kuehm, O.P., Hayden, J.A., & Bearne, S.L.* (2023) A phenylboronic acid-based transition state analogue yields nanomolar inhibition of mandelate racemase. Biochemistry 62, 1929-1942. [Article] [PubMed]
- Bearne, S.L.*, & Hayden, J.A. (2023) Application of circular dichroism-based assays to racemases and epimerases: Recognition and catalysis of reactions of chiral substrates by mandelate racemase. Methods Enzymol. 685, 127-169. [Article] [PubMed]
- McLeod, M.J., Tran, N., McCluskey, G.D., Gillis, T.D., Bearne, S.L.*, & Holyoak, T.* (2023) A metal-dependent conformational change provides a structural basis for the potent inhibition of Escherichia coli CTP synthase by gemcitabine-5'-triphosphate, Protein Sci. 32, e4608. [Article] [PubMed]
- Richards, N. G. J.*, Bearne, S. L., Goto, Y., & Parker, E. J. (2023). Reactivity and mechanism in chemical and synthetic biology. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 378(1871), 20220023. [Article] [PubMed]
- Bearne S. L.* (2023). Capturing the free energy of transition state stabilization: Insights from the inhibition of mandelate racemase. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 378(1871), 20220041. [Article] [PubMed]
- Gillis, T. D., & Bearne, S. L.* (2022). Effects of the 5'-triphosphate metabolites of ribavirin, sofosbuvir, vidarabine, and molnupiravir on CTP synthase catalysis and filament formation: Implications for repurposing antiviral agents against SARS-CoV-2. ChemMedChem, 17(23), e202200399. [Article] [PubMed]
- Pringle, E. S., Duguay, B. A., Bui-Marinos, M. P., Mulloy, R. P., Landreth, S. L., Desireddy, K. S., Dolliver, S. M., Ying, S., Caddell, T., Tooley, T. H., Slaine, P. D., Bearne, S. L., Falzarano, D., Corcoran, J. A., Khaperskyy, D. A.*, & McCormick, C.* (2022). Thiopurines inhibit coronavirus Spike protein processing and incorporation into progeny virions. PLoS Pathogens, 18(9), e1010832. [Article] [PubMed]
- Bearne S.L.*, Guo C.J., Liu J.L.* (2022) GTP-Dependent regulation of CTP synthase: Evolving insights into allosteric activation and NH3 translocation. Biomolecules. 12(5):647. [Article] [PubMed]
- Nagar M., Hayden J.A., Sagey E., Worthen G., Park M., Sharma A.N., Fetter C.M., Kuehm O.P., Bearne S.L.* (2022) Altering the binding determinant on the interdigitating loop of mandelate racemase shifts specificity towards that of D-tartrate dehydratase. Arch Biochem Biophys. 718, 109119. [Article] [PubMed]
- Douglas C.D., Grandinetti L., Easton N.M., Kuehm O.P., Hayden J.A., Hamilton M.C., St. Maurice M., Bearne S.L.* (2021) Slow-onset, potent inhibition of mandelate racemase by 2-formylphenylboronic acid. An unexpected adduct clasps the catalytic machinery. Biochemistry. 60, 2508-2518. [Article] [PubMed]
- Sharma, A.N., Grandinetti, L., Johnson, E.R., St. Maurice, M., and Bearne, S.L.*, (2020) Potent inhibition of mandelate racemase by boronic acids: Boron as a mimic of a carbon acid center. Biochemistry 59:3026-3037 [PubMed]
- Bearne, S.L.*, (2020) Through the looking glass: Chiral recognition of substrates and products at the active sites of racemases and epimerases Eur. J. Chem. 26:10367-10390 [PubMed]
- Harty, M.L., Sharma, A.N., and Bearne, S.L.*, (2019) Catalytic properties of the metal ion variants of mandelate racemase reveal alterations in the apparent electrophilicity of the metal cofactor Metallomics 11:707-723 [PubMed]
- Fetter, C.M., Morrison, Z.A., Nagar, M., Douglas, C.D., and Bearne, S.L.*, (2019) Altering the Y137-K164-K166 triad of mandelate racemase and its effect on the observed pKa of the Brønsted base catalysts Arch. Biochem. Biophys. 666:116-126
- Bearne, S.L.*, (2019) The role of Brønsted base basicity in estimating carbon acidity at enzyme active sites: a caveat Org. Biomol. Chem. 17:7161-7165
- Nasr, M.A., Dovbeshko, G..I, Bearne, S.L., El-Badri, N., and Matta, C.F.*, (2019) Heat shock proteins in the "hot" mitochondrion: Identity and putative roles Bioessays 41:e1900055 [PubMed]
- Sorbara, N.T., MacMillan, J.W.M., McCluskey, G.D., and Bearne, S.L.*, (2019) Substrate-product analogue inhibitors of isoleucine 2-epimerase from Lactobacillus buchneri by rational design Org. Biomol. Chem. 17:8618-8627 [PubMed]
- Easton, N.M., Aboushawareb, S.A.E., and Bearne, S.L.*, (2018) A continuous assay for L-talarate/galactarate dehydratase using circular dichroism Anal. Biochem. 544:80-86 [PubMed]
- McCluskey, G.D., and Bearne, S.L.*, (2018) Biophysical analysis of bacterial CTP synthase filaments formed in the presence of the chemotherapeutic metabolite gemcitabine-5'-triphosphate J. Mol. Biol. 430:1201-1217 [PubMed]
- Pal, M., Easton, N.M., Yaphe, H., and Bearne, S.L.*, (2018) Potent dialkyl substrate-product analogue inhibitors and inactivators of α-methylacyl-coenzyme A racemase from Mycobacterium tuberculosis by rational design Bioorg. Chem. 77:640-650 [PubMed]
- Nagar, M., Kumar, H., and Bearne, S.L.*, (2018) A platform for chemical modification of mandelate racemase: characterization of the C92S/C264S and γ-thialysine 166 variants Protein Eng. Des. Sel. 31:135-145 [PubMed]
- Mackie, J., Kumar, H., and Bearne, S.L.* , (2018) Changes in quaternary structure cause a kinetic asymmetry of glutamate racemase-catalyzed homocysteic acid racemization FEBS Lett. 592:3399-3413 [PubMed]
- McCluskey, G.D., and Bearne S.L.* (2018) "Pinching" the ammonia tunnel of CTP synthase unveils coordinated catalytic and allosteric-dependent control of ammonia passage. Biochim Biophys Acta Gen Subj. 1862:2714-2727
- McCluskey, G., and Bearne, S.L.* , (2018) Anfractuous assemblies of IMP dehydrogenase and CTP synthase: new twists on regulation FEBS J. 285:3724-3728 [PubMed]
- Bearne, S.L.* (2017) The interdigitating loop of the enolase superfamily as a specificity binding determinant or "flying buttress". Biochim Biophys Acta 1865:619-630 [PubMed]
- Bhar, P., and Bearne, S.L.*, (2017) Unexpected side product formed during LDA-induced phosphonylation of uridine Chem. Lett. 46:609-611
- Bearne, S.L.*, and St. Maurice, M.* (2017) A paradigm for CH bond cleavage: Structural and functional aspects of transition state stabilization by mandelate racemase. Adv Protein Chem Struct Biol. 109:113-160 [PubMed]
- Harty, M., and Bearne, S.L.*, (2016) Measuring benzohydroxamate complexation with Mg2+, Mn2+, Co2+, and Ni2+ using isothermal titration calorimetry J. Therm. Anal. Calorim. 123:2573-2582
- Pal, M., Khanal, M., Marko, R., Thirumalairajan, S., and Bearne, S.L.*, (2016) Rational design and synthesis of substrate‐product analogue inhibitors of α‐methylacyl‐coenzyme A racemase from Mycobacterium tuberculosis Chem. Commun. 52:2740-2743 [PubMed]
- McCluskey, G.D., Mohamady, S., Taylor, S.D., Bearne, S.L.*, (2016) Exploring the potent inhibition of CTP synthase by gemcitabine-5'-triphosphate ChemBioChem 17:2240-2249 [PubMed]
- Nagar, M., Wyatt, B.N., St. Maurice, M., Bearne, S.L.*, (2015) Inactivation of mandelate racemase by 3-hydroxypyruvate reveals a potential mechanistic link between enzyme superfamilies Biochemistry 54:2747-2757 [PubMed]
- Nagar, M., Bearne, S.L.*, (2015) An additional role for the Brønsted acid-base catalysts of mandelate racemase in transition state stabilization Biochemistry 54:6743-6752 [PubMed]
- Bearne, S.L.*, (2015) Comment on "A better magnetic stir bar retriever". J. Chem. Educ. 92:1777-1777 [Article]
- Bearne, S.L.*, (2014) Illustrating the effect of pH on enzyme activity using Gibbs energy profiles J. Chem. Educ. 91:84-90
- Harty, M., Nagar, M., Atkinson, L., LeGay, C.M., Derksen, D.J.*, Bearne, S.L.*, (2014) Inhibition of serine and proline racemases by substrate-product analogues Bioorg. Med. Chem. Lett. 24:390-393. [PubMed]
- Pal, M., and Bearne, S.L.*, (2014) Inhibition of glutamate racemase by substrate-product analogues Bioorg. Med. Chem. Lett. 24:1432-1436 [PubMed]
- Nagar, M., Lietzan, A.D., St. Maurice, M., Bearne, S.L.*, (2014) Potent inhibition of mandelate racemase by a fluorinated substrate-product analogue with a novel binding mode Biochemistry 53:1169-1178 [PubMed]
- Pal, M., and Bearne S.L.*, (2014) Synthesis of coenzyme A thioesters using methyl acyl phosphates in an aqueous medium Org. Biomol. Chem. 12:9760-9763 [PubMed]
- Bearne, S.L.*, (2012) Illustrating enzyme inhibition using free energy profiles J. Chem. Educ. 89:732-737.
- Lietzan, A.D., Nagar, M., Pellmann, E.A., Bourque, J.R., Bearne, S.L., St. Maurice, M.*, (2012) Structure of mandelate racemase with bound intermediate analogues benzohydroxamate and cupferron. Biochemistry 51 (6):1160-1170 [PubMed]
- Steeves, C.H., Potrykus, J., Barnett, D.A., and Bearne, S.L.*, (2011) Oxidative stress response in the opportunistic oral pathogen Fusobacterium nucleatum Proteomics 11:2027-2037. [PubMed]
- Steeves, C.H., and Bearne, S.L.*, (2011) Activation and inhibition of CTP synthase from Trypanosoma brucei, the causative agent of African sleeping sickness Bioorg. Med. Chem. Lett. 21:5188-5190. [PubMed]
- Nagar, M., Narmandakh, A., Khalak, Y., Bearne, S.L.*, (2011) Redefining the minimal substrate tolerance of mandelate racemase. Racemization of trifluorolactate. Biochemistry 50(41):8846-8852 [PubMed]
- Narmandakh, A., and Bearne, S.L.*, (2010) Purification of recombinant mandelate racemase: Improved catalytic activity. Protein Expr. Purif. 69:39-46. [PubMed]
- Ouazia, D., and Bearne, S.L.*, (2010) A continuous assay for α-methylacyl-coenzyme A racemase using circular dichroism. Anal. Biochem. 398:45-51. [PubMed]
- Roy, A.C., Lunn, F.A., and Bearne, S.L., (2010) Inhibition of CTP synthase from Escherichia coli by xanthines and uric acids Bioorg. Med. Chem. Lett. 20:141-144. [PubMed]
- Thirumalairajan, S., Mahaney, B., and Bearne, S.L.*, (2010) Interrogation of the active site of OMP decarboxylase from Escherichia coli with a substrate analogue bearing an anionic group at C6 Chem. Comm. 46:3158-3160. [PubMed]
- Potrykus, J., Flemming, J., and Bearne, S.L.*, (2009) Kinetic characterization and quaternary structure of glutamate racemase from the periodontal anaerobe Fusobacterium nucleatum Arch. Biochem. Biophys. 491:16-24. [PubMed]
- Bourque, J.R., and Bearne, S.L.*, (2008) Mutational Analysis of the Active Site Flap (20s Loop) of Mandelate Racemase. Biochemistry 47:566-578. [PubMed]
- Lunn, F.A., MacDonnell, J.E., and Bearne, S.L.*, (2008) Structural requirements for the activation of Escherichia coli CTP synthase by the allosteric effector GTP are stringent, but requirements for inhibition are lax. J. Biol. Chem. 283:2010-2020. [PubMed]
- Taylor, S.D.*, Mirzaei, F., and Bearne, S.L., (2008) Bismethylene Triphosphate Nucleotides of Uridine 4-Phosphate Analogues: A New Class of Anionic Pyrimidine Nucleotide Analogues J. Org. Chem. 73:1403-1412. [PubMed]
- Lunn, F.A., MacLeod, T.J., Bearne, S.L.*, (2008) Mutational analysis of conserved glycine residues 142, 143 and 146 reveals Gly-142 is critical for tetramerization of CTP synthase from Escherichia coli. Biochem. J. 412:113-121. [PubMed]
- Potrykus, J., White, R.L.*, and Bearne, S. L.*, (2008) Proteomic Investigation of Amino Acid Catabolism in the Indigenous Gut Anaerobe Fusobacterium varium. Proteomics 8:2691-2703. [PubMed]
- Taylor, S.D.*, Lunn, F.A., and Bearne, S.L.*, (2008) Ground State, Intermediate, and Multivalent Nucleotide Analogue Inhibitors of Cytidine 5'-Triphosphate Synthase ChemMedChem 3:1853-1857. [PubMed]
- Bourque, J.R., Burley, R.K.M., and Bearne, S.L.*, (2007) Intermediate analogue inhibitors of mandelate racemase: N-Hydroxyformanilide and cupferron. Bioorg. Med. Chem. Lett. 17:105-108. [PubMed]
- Potrykus, J., Mahaney, B., White, R.L.*, and Bearne, S.L.*, (2007) Proteomic investigation of glucose metabolism in the butyrate-producing gut anaerobe Fusobacterium varium. Proteomics 7:1839-1853. [PubMed]
- Taylor, S.D.*, Mirzaei, F., and Bearne, S.L., (2006) An unsymmetrical approach to the synthesis of bismethylene triphosphate analogues. Org. Lett. 8:4243-4246. [PubMed]
- MacLeod, T.J., Lunn, F.A., and Bearne, S.L.*, (2006) The role of lysine residues 297 and 306 in nucleoside triphosphate regulation of E. coli CTP synthase. Inactivation by 2',3'-dialdehyde ATP and mutational analyses. Biochim. Biophys. Acta 1764:199-210. [PubMed]
- Taylor, S.D.*, Mirzaei, F., Sharifi, S., and Bearne, S.L., (2006) Synthesis of Methylene- and Difluoromethylenephosphonate Analogs of Uridine-4-Phosphate and 3-Deazauridine-4-Phosphate. J. Org. Chem. 71:9420-9430. [PubMed]
- O'Malley, P.G.P., Sangster, S.M., Abdelmagid, S.A., Bearne, S.L. and Too, C.K.L.*, (2005) Characterizaion of a novel, cytokine-inducible carboxypeptidase-D isoform in hematopoietic tumor cells. Biochem J. 390:665-673. [PubMed] [Article]
- Bearne, S.L.*, and Spiteri, R.J., (2005) Reduction of intrinsic kinetic and thermodynamic barriers for enzyme-catalysed proton transfers from carbon acid substrates. J. Theor. Biol. 233:563-571. [PubMed]
- Siddiqi, F., Bourque, J.R., Jiang, H., Gardner, M., St. Maurice, M., Blouin, C., and Bearne, S.L.*, (2005) Perturbing the hydrophobic pocket of mandelate racemase to probe phenyl motion during catalysis. Biochemistry 44:9013-9021. [PubMed]
- Brosseau, C.L., St. Maurice, M., Bearne, S.L., and Roscoe, S.G.*, (2005) Electrochemical quartz crystal nanobalance (EQCN) studies of the adsorption behaviour of an enzyme, mandelate racemase, and its substrate, mandelic acid, on Pt. Electrochim. Acta 50:1289-1297.
- Burley, R.K.M., and Bearne, S.L.*, (2005) Inhibition of mandelate racemase by the substrate-intermediate-product analogue 1,1-diphenyl-1-hydroxymethylphosphonate. Bioorg. Med. Chem. Lett. 15:4342-4344. [PubMed]
- Lunn, F.A., and Bearne, S.L.*, (2004) Alternative Substrates for Wild-type and L109A E. coli CTP Synthases. Kinetic Evidence for a Constricted Ammonia Tunnel. Eur. J. Biochem. 271:4204-4212. [PubMed]
- MacDonnell, J.E., Lunn, F.A., and Bearne, S.L.*, (2004) Inhibition of E. coli CTP synthase by the positive allosteric effector GTP. Biochim. Biophys. Acta 1699:213-220. [PubMed]
- St. Maurice, M., and Bearne, S.L.*, (2004) Hydrophobic nature of the active site of mandelate racemase. Biochemistry 43:2524-2532. [PubMed]
- Iyengar, A., and Bearne, S.L.*, (2003) Aspartate-107 and leucine-109 facilitate efficient coupling of glutamine hydrolysis to CTP synthesis by Escherichia coli CTP synthase. Biochem. J. 369:497-507. [PubMed]
- Simard. D., Hewitt, K.A., Lunn, F., Iyengar, A., and Bearne, S.L.*, (2003) Limited proteolysis of Escherichia coli cytidine 5'-triphosphate synthase. Identification of residues required for CTP formation and GTP-dependent activation of glutamine hydrolysis. Eur. J. Biochem. 270:2195-2206. [PubMed]
- St. Maurice, M., Bearne, S.L.*, Lu, W., and Taylor, S.D., (2003) Inhibition of mandelate racemase by α-fluorobenzylphosphonates. Bioorg. Med. Chem. Lett. 13:2041-2044. [PubMed]
- St. Maurice, M., and Bearne, S.L.*, (2002) Kinetics and thermodynamics of mandelate racemase catalysis. Biochemistry 41:4048-4058. [PubMed]
- Iyengar, A., and Bearne, S.L.*, (2002) An assay for cytidine 5'-triphosphate synthetase glutaminase activity using high performance liquid chromatography. Anal. Biochem. 308:396-400. [PubMed]
- Bearne, S.L.*, White, R.L.*, MacDonnell, J.E., Bahrami, S., and Gronlund, J., (2001) Purification and characterization of beta-methylaspartase from Fusobacterium varium. Mol. Cell. Biochem. 221:117-126. [PubMed]
- Bearne, S.L.*, Hekmat, O., and MacDonnell, J.E., (2001) Inhibition of Escherichia coli CTP synthase by glutamate γ-semialdehyde and the role of the allosteric effector GTP in glutamine hydrolysis. Biochem. J. 356:223-232. [PubMed]
- St. Maurice, M., and Bearne, S.L.*, (2000) Reaction intermediate analogues for mandelate racemase: interaction between Asn 197 and the α-hydroxyl of the substrate promotes catalysis. Biochemistry 39:13324-13335. [PubMed]
- Bearne, S.L.*, and Blouin, C., (2000) Inhibition of Escherichia coli glucosamine-6-phosphate synthase by reactive intermediate analogues. The role of the 2-amino function in catalysis. J. Biol. Chem. 275:135-140. [PubMed]
- Bearne, S.L.*, St. Maurice, M., and Vaughan, M.D., (1999) An assay for mandelate racemase using high-performance liquid chromatography. Anal. Biochem. 269:332-336. [PubMed]