David M. Waisman
Professor

Email: david.waisman@dal.ca
Phone: 902-494-1803
Phone: 902-494-2766 (lab)
Mailing Address:
Sir Charles Tupper Medical Building
PO Box 15000
Halifax, Nova Scotia, Canada B3H 4R2
Education
- PhD, University of Manitoba
Academic Positions
- Department member since 2006
- Canada Research Chair in Cancer Research
Research Topics
Mechanisms of Tumor Growth, Invasiveness and Metastases
Research
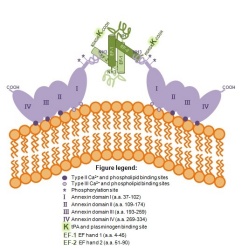
Role of S100A10 in tumor growth, invasion and metastasis.
The defining characteristic of a tumor cell is its ability to escape the constraints imposed by neighboring cells and membranes, invade the surrounding tissue and metastasize to new sites in distant tissues. This invasive property of tumor cells involves attachment, localized proteolysis and migration through the proteolytically modified surrounding tissue. This localized proteolysis is dependent on activation of proteases at the cell surface or the release of the proteinases from tumor cells and tumor-associated cells. One of the major proteinases thought to participate in the pericellular proteolysis associated with the invasive program of tumor cells is plasmin. Plasmin is a fundamentally important enzyme involved in physiological processes such as blood clot lysis and pathological processes such as tumor growth and metastasis. Recent work from our laboratory has shown that the protein, S100A10, regulates the production and activity of plasmin at the cell surface. Furthermore, using several animal models of cancer, we have shown that S100A10 regulates tumor growth, invasiveness and metastasis. We are utilizing biochemical, cellular and molecular biological approaches to study the mechanism by which S100A10 regulates plasmin and contributes to tumor growth and as well as the metastatic potential of cancer cells. Our findings will provide a deeper understanding of the cancer cell invasion process thereby allowing for future pharmacotherapeutic protease inhibitor design and for future translational research to prevent the escape of cancer cells from the tumor thus prolonging patient survival.
A catastrophic collaboration; S100A10 facilitates the tumor promoting association of macrophages with tumor cells
Macrophages also play a fundamental role as mediators of tumor growth, invasion and metastasis. The movement of macrophages across the basement membrane and through the extracellular matrix to the tumor site requires the activation of proteases, such as plasmin, at their cell surface. Recently, we reported that the growth of murine Lewis lung carcinoma or T241 fibrosarcoma was dramatically reduced in S100A10-null mice compared to wild-type mice. The tumor growth deficit corresponded with a decrease in macrophage density, suggesting that macrophages required S100A10 to migrate to the tumor site. Although the tumor growth deficit observed in S100A10-null mice was restored by intraperitoneal injection of WT macrophages, S100A10-null macrophages only restored tumor growth when directly injected into the tumor, suggesting a loss in migratory capability by S100A10-null macrophages. Lastly, selective depletion of macrophages from wild-type mice resulted in similar tumor growth deficits to that in the S100A10-null mice. Our study showed that S100A10 was required for macrophage migration to the tumor site and that this represents a rate limiting step in tumor progression. Our results highlight a new role for the S100A10 protein in the recruitment of macrophages to the tumor site and show for the first time that macrophages require S100A10-mediated plasmin generation to facilitate their movement to the tumor site. We are currently using biochemical and molecular biological techniques to establish the role that S100A10 plays in macrophage migration and explain why S100A10-mediated migration is a rate limiting step in tumor formation. We are also defining the role that S100A10 plays in the initiation of metastatic foci by macrophages.
Regulation of fibrinolysis and angiogenesis by S100A10
Endothelial cells form the inner lining of vascular networks and maintain blood fluidity by inhibiting blood coagulation and promoting blood clot dissolution (fibrinolysis). An inability to turn on plasmin generation and digest blood clots leads to heart attack and stroke. We have shown that compared with wild-type mice, S100A10-null mice displayed increased deposition of fibrin in the vasculature and reduced clearance of vascular thrombi, suggesting a role for S100A10 in fibrinolysis in vivo. Compared to wild-type, endothelial cells from S100A10-null mice demonstrated a 40% reduction in plasminogen binding and plasmin generation in vitro. Furthermore, S100A10-deficient endothelial cells demonstrated impaired neovascularization of Matrigel plugs in vivo suggesting a role for S100A10 in angiogenesis. This study established an important role for S100A10 in the regulation of fibrinolysis and angiogenesis in vivo, and suggested that S100A10 plays a critical role in endothelial cell function. We are characterizing the role of S100A10 in fibrinolysis and angiogenesis using in vitro analysis and animal models.
Keywords:
mouse models of cancer, tumor growth, angiogenesis, cell culture, small interfering RNA, tumor imaging, cancer cell invasion, proteolysis.
Current Lab Members
| Alamelu (Dharini) Bharadwaj | Postdoc (University of Nebraska, USA) |
|---|---|
| Moamen Bydoun |
Grad Student (PhD, Pathology) |
| Ludovic Durrieu | Postdoc (Canada) |
Publications
- Madureira PA, Hill R, Miller VA, Giacomantonio C, Lee PW, Waisman DM., (2011) Annexin A2 is a novel Cellular Redox Regulatory Protein involved in Tumorigenesis Oncotarget 2 (12):1075-1093 [PubMed]
- Hill R, Madureira PA, Waisman DM, Lee PW., (2011) DNA-PKCS binding to p53 on the p21WAF1/CIP1 promoter blocks transcription resulting in cell death Oncotarget 2 (12):1094-1108 [PubMed]
- O'Connell PA, Madureira PA, Berman JN, Liwski RS, Waisman DM, (2011) Regulation of S100A10 by the PML-RAR-α oncoprotein Blood 117(15):4095-105 [PubMed]
- Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM, (2011) Regulation of fibrinolysis by S100A10 in vivo. Blood 118(11):3172-81 [PubMed]
- Phipps KD, Surette AP, O'Connell PA, Waisman DM, (2011) Plasminogen Receptor S100A10 Is Essential for the Migration of Tumor-Promoting Macrophages into Tumor Sites Cancer Research 71(21):6676-6683 [PubMed]
- Madureira PA, Surette AP, Phipps KD, Taboski MA, Miller VA, Waisman DM, (2011) The role of the annexin A2 heterotetramer in vascular fibrinolysis Blood 118(18):4789-97 [PubMed]
- O'Connell, P. A., Surette, A. P., Liwski, R. S., Svenningsson, P., and Waisman, D. M., (2010) S100A10 regulates plasminogen-dependent macrophage invasion Blood 116(7):1136-1146 [PubMed]
- Zhang Y, Schulte W, Pink D, Phipps K, Zijlstra A, Lewis JD, Waisman DM, (2010) Sensitivity of cancer cells to truncated diphtheria toxin PLoS One 5(5):e10498 [PubMed]
- Weber TJ, Opresko LK, Waisman DM, Newton GJ, Quesenberry RD, Bollinger N, Moore RJ, Smith RD, (2009) Regulation of the low-dose radiation paracrine-specific anchorage-independent growth response by annexin A2 Radiat Res 172(1):96-105 [PubMed]
- Hill R, Leidal AM, Madureira PA, Gillis LD, Cochrane HK, Waisman DM, Chiu A, Lee PW, (2008) Hypersensitivity to chromium-induced DNA damage correlates with constitutive deregulation of upstream p53 kinases in p21-/- HCT116 colon cancer cells DNA Repair (Amst) 7(2):239-52 [PubMed]
- Hill R, Leidal AM, Madureira PA, Gillis LD, Waisman DM, Chiu A, Lee PW, (2008) Chromium-mediated apoptosis: involvement of DNA-dependent protein kinase (DNA-PK) and differential induction of p53 target genes DNA Repair (Amst) 7(9):1484-99 [PubMed]
- Derry MC, Sutherland MR, Restall CM, Waisman DM, Pryzdial EL., (2007) Annexin 2-mediated enhancement of cytomegalovirus infection opposes inhibition by annexin 1 or annexin 5 J Gen Virol. 88:19-27 [PubMed]
- Shao C, Zhang F, Kemp MM, Linhardt RJ, Waisman DM, Head JF, Seaton BA., (2006) Crystallographic analysis of calcium-dependent heparin binding to annexin A2 J Biol Chem. 20:31689-31695 [PubMed]
- Waisman, D. M., (2005) Annexin A2 may not play a role as a plasminogen receptor. Br.J.Haematol. 131:553-554 [PubMed]
- Waisman, D. M., (2005) Characterization of the RNA-Binding Properties of Annexin A2 Heterotetramer. Annexins 1:191-199
- MacLeod, T. J. N. R. Filipenko & D. M. Waisman., (2005) Characterization of the RNA-Binding Properties of Annexin A2 Heterotetramer. Annexins 1:191-199
- Kwon, M. T. J. MacLeod Y. Zhang & D. M. Waisman., (2005) S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Front Biosci. 10:300-325 [PubMed]
- Kwon, M. C. S. Yoon W. Jeong S. G. Rhee & D. M. Waisman., (2005) Annexin A2-S100A10 heterotetramer, a novel substrate of thioredoxin. J.Biol.Chem. 280:23584-23592 [PubMed]
- Zhao, W. Q. D. M. Waisman & M. Grimaldi., (2004) Specific localization of the annexin II heterotetramer in brain lipid raft fractions and its changes in spatial learning. J. Neurochem. 90:609-620 [PubMed]
- Zhang, L. D. K. Fogg & D. M. Waisman., (2004) RNA interference-mediated silencing of the S100A10 gene attenuates plasmin generation and invasiveness of Colo 222 colorectal cancer cells. J.Biol.Chem. 279:2053-2062 [PubMed]
- Filipenko, N. R. T. J. MacLeod C. S. Yoon & D. M. Waisman., (2004) Annexin A2 is a novel RNA-binding protein. J. Biol. Chem. 279:8723-8731 [PubMed]
- Caplan, J. F. N. R. Filipenko S. L. Fitzpatrick & D. M. Waisman., (2004) Regulation of annexin A2 by reversible glutathionylation. J.Biol.Chem. 279:7740-7750 [PubMed]
- Barabas, A. Z. C. D. Cole A. D. Barabas J. M. Cowan C. S. Yoon D. M. Waisman & R. Lafreniere., (2004) Presence of immunoglobulin M antibodies around the glomerular capillaries and in the mesangium of normal and passive Heymann nephritis rats. Int.J.Exp.Pathol. 85:201-212 [PubMed]
- Waisman, D. M., (2003) Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J.Biol.Chem. 278:25577-25584 [PubMed]
- Le, D. M. A. Besson D. K. Fogg K. S. Choi D. M. Waisman C. G. Goodyer B. Rewcastle & V. W. Yong., (2003) Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J. Neurosci. 23:4034-4043 [PubMed]
- Kwon, M. & D.M. Waisman, (2003) Mechanism of angiostatin formation from plasminogen. Plasminogen: Structure, Activation, and Regulation Eds. Waisman, D. M. Kluwer academic/plenum publishers New York 8,:135-156
- Waisman, D. M., (2003) Plasminogen Receptors Plasminogen:Structure, Activation and Regulation/. Eds. Waisman, D. M. luwer Academic/Plenum publishers 5:81-101
- Filipenko, N.R. & D.M. Waisman, (2003) Annexin II- Analysis of a pleiotropic protein. In Annexins: Biological importance and annexin-related pathologies. Bioscience:127-156
- Choi, K.S., D.K. Fogg, S.L. Fitzpatrick & D.M. Waisman, (2003) Role of annexin II tetramer in the regulation of plasmin activity. Annexins: Biological importance and annexin-related pathologies. Eds. Joanna Bandorowicz-Pikula Ed Landes Bioscience:218-233
- Choi, K. S. D. K. Fogg C. S. Yoon & D. M. Waisman., (2003) P11 regulates extracellular plasmin production and invasiveness of HT1080 fibrosarcoma cells. FASEB J 17:235-246
- Kwon, M. J. F. Caplan N. R. Filipenko K. S. Choi S. L. Fitzpatrick L. Zhang & D. M. Waisman., (2002) Identification of Annexin II Heterotetramer as a Plasmin Reductase. J.Biol.Chem. 277:10903-10911 [PubMed]
- Kang, H. M. N. R. Filipenko G. Kassam & D. M. Waisman., (2002) Recombinant annexin II tetramer. Methods Mol.Biol 172:225-234 [PubMed]
- Fogg, D. K. D. E. Bridges K. T. Cheung G. Kassam N. R. Filipenko K. S. Choi S. L. Fitzpatrick M. Nesheim & D. M. Waisman., (2002) The p11 subunit of annexin II heterotetramer is regulated by basic carboxypeptidase. Biochemistry 41:4953-4961
- Brooks, N. D. J. E. Grundy N. Lavigne M. C. Derry C. M. Restall C. R. MacKenzie D. M. Waisman & E. L. Pryzdial., (2002) Ca2+-dependent, phospholipid-independent binding of annexin 2 to annexin 5. Biochem.J. 367:895-900 [PubMed]
- Kwon, M. C. S. Yoon S. Fitzpatrick G. Kassam K. S. Graham M. K. Young & D. M. Waisman., (2001) p22 is a novel plasminogen fragment with antiangiogenic activity. Biochemistry 40:13246-13253 [PubMed]
- Kassam, G. M. Kwon C. S. Yoon K. S. Graham M. K. Young S. Gluck & D. M. Waisman., (2001) Purification and characterization of A_61 ,.an angiostatin-like plasminogen fragment produced by plasmin autodigestion in the absence of sulfhydryl donors J.Chem.Biol. 276:8924-8933 [PubMed]
- Filipenko, N. R. and D. M. Waisman., (2001) The C-terminus of annexin II mediates binding to f-actin. J.Biol.Chem. 276:5310-5315 [PubMed]
- Filipenko, N. R. H. M. Kang & D. M. Waisman., (2001) Characterization of the Ca2+-binding sites of annexin II tetramer. J.Biol.Chem. 276:3718-PM [PubMed]
- Choi, K. S. S. L. Fitzpatrick N. R. Filipenko D. K. Fogg G. Kassam A. M. Magliocco & D. M. Waisman., (2001) Regulation of plasmin-dependent fibrin clot lysis by annexin II heterotetramer. J.Biol.Chem. 276:25212-25221 [PubMed]
- Mai, J. D. M. Waisman & B. F. Sloane., (2000) Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim.Biophys.Acta 1477:215-230 [PubMed]
- Mai, J. R. L. Finley, Jr. D. M. Waisman & B. F. Sloane., (2000) Human Procathepsin B Interacts with the Annexin II Tetramer on the Surface of Tumor Cells. J.Biol.Chem. 275:12806-12812 [PubMed]
- Fitzpatrick, S. L. G. Kassam K. S. Choi H.-M. Kang Fogg.D.K. & D. M. Waisman., (2000) Regulation of plasmin activity by annexin II tetramer. Biochemistry 39:1021-1028
- Fitzpatrick, S. L. G. Kassam A. Manro C. E. Braat P. Louie & D. M. Waisman., (2000) Fucoidan-Dependent Conformational Changes in Annexin II Tetramer. Biochemistry 39:2140-2148