Roy Duncan
Professor

Email: roy.duncan@dal.ca
Phone: 902-494-6770
Mailing Address:
Sir Charles Tupper Medical Building
PO Box 15000
Halifax, Nova Scotia, Canada B3H 4R2
Education
- PhD, University of Guelph
Academic Positions
- Department member since 2010
- Killam Chair in Virology
- Fusogenix Inc., Founder and CEO
Research Topics
Protein-mediated cell-cell membrane fusion during viral infections and muscle cell differentiation; viral fusion protein structure and function; cellular pathways involved in cell-cell fusion; exosomes and nonenveloped virus transmission; oncolytic viruses for tumour therapy.
Research
Cell-Cell Membrane Fusion
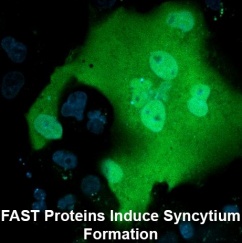
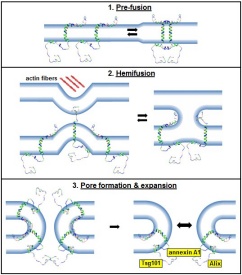
Cell-cell membrane fusion is involved in numerous biological processes, including fertilization (sperm-egg fusion), muscle (myotubes) and placenta (syncytiotrophoblast) formation, bone maintenance (osteoclasts), and possibly cancer cell metastasis (reviewed in Developmental Cell 14: 11-21, 2008). The cellular proteins and pathways responsible for these cell-cell fusion events remain elusive. My research group discovered a new family of virus-encoded cellular fusogens. The reovirus Fusion-Associated Small Transmembrane (FAST) proteins are the smallest known membrane fusion proteins, and are the only identified fusion proteins from nonenveloped viruses. Rather than inducing virus-cell fusion, these non-structural viral proteins are expressed inside virus-infected cells where they traffic to the plasma membrane and induce cell-cell fusion resulting in multinucleated syncytium formation. From a virus perspective, the FAST proteins promote rapid dissemination of the infection and contribute to the inherent pathogenicity of the fusogenic reoviruses. These virus-encoded cellular fusogens provide a new model to investigate the mechanisms responsible for protein-mediated cell-cell membrane fusion. Studies to date indicate the FAST proteins do not function according to the paradigm established from investigations of the fusion proteins encoded by enveloped viruses such as HIV and influenza virus. Using fluorescent fusion assays (FACS analysis and confocal microscopy), site-directed mutagenesis, and proteomic approaches, we are exploring both the nature of the FAST proteins and the cellular proteins and pathways involved in cell-cell fusion events.
Membrane Protein Structure and Function
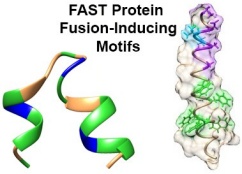
Current models of protein-mediated membrane fusion are based extensively on the complex, multimeric fusion proteins encoded by enveloped viruses that mediate virus-cell fusion. Since the FAST proteins mediate cell-cell, rather than virus-cell, membrane fusion, their mode of action may be more relevant to the undefined proteins responsible for biological cell-cell fusion events. Using CD and NMR spectroscopy coupled with liposome-cell, cell-cell, and peptide-induced liposome-liposome fusion assays, we are pursuing a mechanistic analysis of how dynamic changes in FAST protein fusion motifs allow the FAST proteins to function as modular cell-cell fusogens.
Cellular Pathways for Cell-Cell Fusion
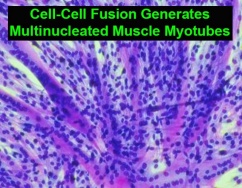
While FAST proteins are both necessary and sufficient to fuse lipid bilayers, they rely on and recruit cellular co-factors to assist with the more complex process of fusing cells together and generating syncytia. Interestingly, we discovered that some of these co-factors identify cellular processes that are central to the general process of cell-cell fusion mediated by diverse viral and cellular fusogens. Using FAST proteins, enveloped virus fusion proteins, and differentiation-dependent fusion of muscle cells to generate multinucleated muscle myotubes, we are determining how these pathways are interconnected and how they contribute to syncytiogenesis.
Protein Trafficking and Unconventional Secretion Pathways
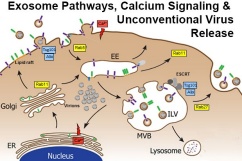
Membrane proteins must be routed to the correct cellular membrane compartment, which requires specific interactions between sorting and trafficking signals in the membrane protein with the complex, multi-protein complexes that regulate vesicular transport. Using reovirus FAST proteins as our model, we are exploring the function of a novel Golgi export signal in recruiting Rab-dependent trafficking pathways. These studies have led to pathways that promote release of extracellular vesicles (exosomes) and unconventional release of nonenveloped viruses.
Anti-Cancer Drug Delivery
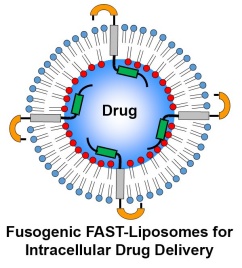
Systemic distribution of anti-cancer drugs contributes to the severe toxic side effects associated with chemotherapy. Encapsulation of these drugs inside liposomes can restrict their biodistribution and reduce these adverse reactions. However, the drug must still be delivered from inside the liposome to inside the cancer cell. Using fusogenic liposomes containing the FAST proteins, we can promote liposome-cell fusion and increased intracellular drug delivery. Working in collaboration with a private sector partner, we are now developing and evaluating FAST-liposomes that specifically target the leaky vasculature of tumours.
Cancer Cell Migration and Invasion
Cancer metastasis requires numerous changes to convert an adherent tumour cell to one capable of migration and invasion into the circulatory system. Many of these changes involve altered cytoskeletal-membrane interactions. We recently discovered that the FAST proteins interact with myopodin, an actin bundling protein whose loss correlates with invasive prostate cancer. We also discovered that myopodin expression promotes muscle cell fusion, suggesting a convergence of cellular pathways involved in cell-cell fusion and tumour invasion. Using RNAi and proteomic approaches with prostate and muscle cell lines expressing myopodin, we are examining the basis for how myopodin promotes cell fusion, migration and invasion.
Current Lab Members
| Roberto de Antueno |
Senior Research Associate |
|---|---|
| Gerard Gaspard | Postdoc (India, Dalhousie University) |
| Han He | Technician |
| Duncan MacKenzie |
Grad Student (Micro & Immuno) |
| Nandini Margam | Grad Student (Micro & Immuno) |
| Nichole McMullen |
Research Associate & Lab Manager |
| Sandra Mendes | Postdoc (Brazil) |
| Chungen Pan |
Postdoc (University of Peking, New York Blood Center) |
| Hiren Parmar | Postdoc (India, Sweden, Dalhousie University) |
Former Lab Members
| Muzaddid Sarker | 2016 | Research Associate, University of North Carolina |
|---|---|---|
| Hirendrasinh Parmar | 2015 | PhD Program (Dal) |
| Jolene Read | 2014 | Cell Therapy Research Technician, University Health Network, Toronto |
| Marta Ciechonska |
2013 | PDF, Imperial College, London |
| Fui Boon Kai |
2013 | PDF, UCSF |
| Tim Key |
2013 | Manager, Technology & Venture Development, MaRS Innovation |
Publications
- Pandey, A., LeBlanc, D.M., Parmar, H.B., Phạm, T.T.T., Sarker, M., Xu, L., Duncan, R., Liu, X-Q. and Rainey, J.K., (2019) Structure, amphipathy, and topology of the membrane-proximal helix 8 influence apelin receptor plasma membrane localization Biochim Biophys Acta - Biomembranes 1861:180036 [PubMed] [Article]
- Thomas, M.L., de Antueno, R., Coyle, K.M., Sultan, M., Cruickshank, B.M., Giacomantonio MA, Giacomantonio, C.A., Duncan, R., Marcato, P., (2016) Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol Oncol. 10(9):1485-1496
- Parmar, H. and Duncan, R., (2016) A novel tribasic Golgi export signal directs cargo protein interaction with activated Rab11 and AP-1dependent Golgiplasma membrane trafficking Mol. Biol. Cell 27:1320-1331
- Read, J., Clancy, E.K., Sarker, M., de Antueno, R., Langelaan, D.N., Parmar, H.B., Shin, K., Rainey, J.K. and Duncan, R., (2015) Reovirus FAST proteins drive pore formation and syncytiogenesis using a novel helix-loop-helix fusion-inducing lipid packing sensor PLoS Pathog 11(6):e1004962 [PubMed] [Article]
- Key, T., Sarker, M., de Antueno, R., Rainey, J.K. and Duncan, R., (2015) The p10 FAST protein fusion peptide functions as a cystine noose to induce cholesterol-dependent liposome fusion without liposome tubulation. Biochim Biophys Acta - Biomembranes 1848:408-416 [PubMed] [Article]
- Falkenham, A., de Antueno, R., Rosin, N., Betsch, D., Lee, T.D.G., Duncan, R., Légaré, J.-F., (2015) Non-classical resident macrophages are important determinants in the development of myocardial fibrosis. Amer. J. Pathol. 185:927-942
- Kai, F., Fawcett, J., and Duncan, R., (2015) Synaptopodin-2 induces assembly of peripheral actin bundles and immature focal adhesions to promote lamellipodia formation and prostate cancer cell migration Oncotarget 6:11162-11174
- Key, T. and Duncan, R., (2014) A compact, multifunctional fusion module directs cholesterol-dependent homomultimerization and syncytiogenic efficiency of reovirus p10 FAST proteins. PLOS Pathogens 10:e1004023 [PubMed]
- Ciechonska, M., Key, T., and Duncan, R., (2014) Efficient reovirus- and measles virus-mediated pore expansion during syncytium formation is dependent on annexin A1 and intracellular calcium. J. Virol. 88:6137-6147 [PubMed]
- Ciechonska, M. and Duncan, R., (2014) Lysophosphatidylcholine arrests fusion pore expansion following reovirus FAST protein- and influenza virus hemagglutinin-induced cell-cell membrane fusion. J. Virol. 88:6528-6531 [PubMed]
- Ciechonska, M. and Duncan, R. , (2014) Reovirus FAST proteins: virus-encoded cellular fusogens. Trends in Microbiology 22:715-724 [PubMed]
- Duncan, R., J Lewis, R J De Antueno, R-L Nesbitt, E. K. Clancy, (2014) Recombinant polypeptides for membrane fusion and uses thereof. PATENT, Assignee: Innovascreen Inc.:
- Parmar, H., Barry, C., Kai, F., and Duncan, R., (2014) Golgi complex-plasma membrane trafficking directed by an autonomous, tri-basic Golgi export signal Mol. Biol. Cell 25:866-878 [PubMed]
- Parmar, H., Barry, C., and Duncan, R., (2014) Polybasic trafficking signal mediates Golgi export, ER retention or ER export and retrieval based on membrane-proximity PLOS One 9:e94194 [PubMed]
- Key, T., Read, J. A., Nibert, M. L., and Duncan, R., (2013) Piscine reovirus encodes apparent homologs of orthoreovirus outer-clamp and fiber proteins and a novel non-fusogenic cytotoxic integral membrane protein. J. Gen. Virol. 94:1039-1050 [PubMed]
- Nibert, M. L., and Duncan, R., (2013) Bioinformatics of recent aqua- and orthoreovirus isolates from fish: evolutionary gain or loss of FAST and fiber proteins and taxonomic implications PLOS One 8:e68607 [PubMed]
- Kai, F., and Duncan, R., (2013) Prostate cancer cell migration induced by myopodin isoforms is associated with formation of morphologically and biochemically distinct actin networks. FASEB J. 27:5046-5058 [PubMed]
- Top, D., Read, J., Dawe, S., Syvitski, R., and Duncan, R., (2012) Cell-cell membrane fusion induced by the p15 FAST protein requires a novel fusion peptide motif containing a myristoylated polyproline type II helix. J. Biol. Chem. 287:3403-3414 [PubMed]
- Kai, F.B., Tanner, K., King, C., and Duncan, R., (2012) Myopodin isoforms alter the chemokinetic response of PC3 cells in response to different migration stimuli via differential effects on Rho-ROCK signaling pathways. Carcinogenesis 33:2100-2107 [PubMed]
- Corcoran, J., Clancy, E.K., and Duncan, R., (2011) Homomultimerization of the reovirus p14 FAST protein during transit through the ER-Golgi secretory pathway. J. Gen. Virol. 92:162-166 [PubMed]
- Clancy, E.K. and Duncan, R., (2011) Helix-destabilizing, beta-branched and polar residues in the baboon reovirus p15 transmembrane domain influence the modularity of the FAST proteins. J. Virol. 85:4707-4719 [PubMed]
- Yan, X., Parent, K. N., Goodman, R. P., Tang, J., Shou, J., Nibert, M. L., Duncan, R., and Baker, T.S. , (2011) Virion structure of baboon reovirus, a fusogenic orthoreovirus that lacks an adhesion fiber. J. Virol. 85:7483-7495 [PubMed]
- Noyce, R. S., Taylor, K., Ciechonska, M., Collins, S. E., Duncan, R., and Mossman, K. L., (2011) Membrane perturbation elicits an IRF3-dependent IFN-independent antiviral response. J. Virol. 85:10926-10931. [PubMed]
- Read, J. A. and Duncan, R., (2011) Biophysical and functional assays for viral membrane fusion peptides. Methods 55:122-126 [PubMed]
- Racine, R. and Duncan, R., (2010) Facilitated leaky scanning and atypical ribosome shunting direct downstream translation initiation on the tricistronic S1 mRNA of avian reovirus Nucleic Acids Res 38:7260-7272 [PubMed]
- Barry, C., Key, T., Haddad, R., and Duncan, R., (2010) Features of a spatially constrained cystine loop in the p10 FAST protein ectodomain define a new class of viral fusion peptides J. Biol. Chem. 285:16424-16433 [PubMed]
- Clancy, E.K., Barry, C., Ciechonska, M., and Duncan, R., (2010) Different activities of the reovirus FAST proteins and influenza hemagglutinin in cell-cell fusion assays and in response to membrane curvature agents Virology 397:119-129 [PubMed]
- Top, D., Barry, C., Racine, T., Ellis, C., and Duncan, R., (2009) Enhanced fusion pore expansion mediated by the trans-acting endodomain of the reovirus FAST proteins. PLoS Pathogens 5:e1000331 [PubMed]
- Clancy, E. K. and Duncan, R., (2009) Reovirus FAST protein transmembrane domains function in a modular, primary sequence-independent manner to mediate cell-cell membrane fusion. J. Virol. 83:2941-2950 [PubMed]
- Racine, T. Hurst, T., Shou, J., and Duncan, R., (2009) Aquareovirus effects syncytiogenesis using a novel member of the FAST protein family translated from a non-canonical translation start site. J. Virol. 83:5951-5955 [PubMed]
- Brown, C.W., Stephenson, K.B., Hanson, S., Kucharczyk, M., Duncan, R., Bell, J.C., and Lichty, B.D. , (2009) The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J. Virol. 83:552-561 [PubMed]
- Barry, C. and Duncan, R., (2009) Multifaceted sequence-dependent and -independent roles for the reovirus FAST protein cytoplasmic tails in fusion pore formation and syncytiogenesis J. Virol. 83:12185-12195 [PubMed]
- Richardson, A., de Antueno, R., Duncan, R., and Hoskin, D.W., (2009) Intracellular delivery of the antimicrobial core (RRWQWR) of bovine lactoferricin triggers cathepsin B- and caspase-dependent cytotoxicity in Jurkat T-leukemia cells Biochem. Biophys. Res. Comm. 388:736-741 [PubMed]
- Richardson, A., de Antueno, R., Duncan, R., Hoskin, D.W., (2009) Intracellular delivery of bovine lactoferricin s antimicrobial core (RRWQWR) kills T-leukemia cells Biochem. Biophys. Res. Commun. 388:736-741
- Salsman, J., Top, D., Barry, C. and Duncan, R., (2008) A virus-encoded cell-cell fusion machine dependent on surrogate adhesins. PloS Pathogens 4:e1000016 [PubMed]
- Racine, T., Barry, C., Roy, K., Dawe, S. J., Shmulevitz, M., and Duncan, R., (2007) Leaky scanning and scanning-independent ribosome migration on the tricistronic S1 mRNA of avian reovirus. J. Biol. Chem. 282:25613-25622 [PubMed]
- Mader, J. S., Richardson, A., Salsman, J., Top, D., de Antueno, R., Duncan, R. and Hoskin, D. W. , (2007) Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria Exp. Cell Res. 313:2634-2650 [PubMed]
- Mader, J.S., Richardson, A., Salsman, J., Top, D., de Antueno, R., Duncan, R., Hoskin, D.W., (2007) Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria. Exp. Cell. Res. 313:2634-2650
- Corcoran JA, Salsman J, de Antueno R, Touhami A, Jericho MH, Clancy EK, Duncan R., (2006) The p14 fusion-associated small transmembrane (FAST) protein effects membrane fusion from a subset of membrane microdomains. J Biol. Chem. 281:31778-31789
- Top D, de Antueno R, Salsman J, Corcoran J, Mader J, Hoskin D, Touhami A, Jericho MH, Duncan R., (2005) Liposome reconstitution of a minimal protein-mediated membrane fusion machine. EMBO J. 24:2980-2988